Independent Integrity Inspection Limited CIP Validation inspection / Riboflavin test is a test to validate the hygienic design and actual hygienic condition of your equipment.
Within the food, brewing, drink and pharmaceutical industries there is an ever-present risk of product contamination that, if undetected, could result in product recalls, damage to brand names and, in the worst case, serious illness or death to a consumer.
Tests for an examination of cleanability play a major role in sterile process technology. Riboflavin testing provides a solution to validate the effectiveness of the CIP (Cleaning In Place) procedure.
Advantages include:
Validation of CIP installations in new equipment.
Validating the CIP installation as part of FAT ( Factory Acceptance Test ) or upon final installation allow you to avoid design-related hygienic problems and take corrective actions before the start of production. Saving costs and production downtime.
Validation of modifications.
In case of modifications to your equipment, the CIP validation provides certainty that your equipment is cleanable.
Riboflavin testing also provides solutions of what may be causing contamination as testing is the only proven way to efficiently monitor spray coverage, which is needed for a sanitary clean.
Common Issues affecting CIP cleaning.
The most common issues connected with CIP cleaning:
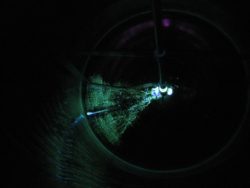
CIP test / Riboflavin testing to highlight problematic areas during the CIP process
- Clogged spray devices;
- Spray patterns that do not meet complex designs;
- Damaged / missing spray devices;
- Incorrectly located spray devices;
- Spray patterns that are not designed to work with customer tank modifications;
- Design, pressure and flow rate inadequate for the design of the equipment;
- Modified equipment needing a new validation;
- Baffles, agitators or other obstructions causing shadowing / difficult to clean areas;
- Miscellaneous mechanical issues.
Independent Integrity Inspection Limited‘s CIP Validation / Riboflavin Test is a fast and accurate test to examine the cleanability in new and operating equipment. A solution containing water and a fluorescent vitamin covers all surfaces within the equipment. The equipment is then flushed by completing the predetermined rinsing cycle.
Critical cleaning points are visible for the highly trained technicians using state of the art innovative fluorescent equipment to verify the cleanability within the equipment.
- Localises critical cleaning points that may cause hygienic risks/design flaws.
- 100% food-grade inspection, only using water and a known vitamin as the test solution.
Full documentation for your quality management.
Highest levels of product purity and quality are of primary importance, particularly in such industries as the beverage, dairy, food and pharmaceutical industry. Independent Integrity Inspection Limited supports you in implementing your quality management routine by providing comprehensive test reports which are produced for every inspection of your spray dryers and associated equipment carried out by our engineers.
Please contact us to find out more about the services we offer or fill in the online enquiry form here:




